-
Please try to be as accurate as possible with your search.
-
We can quote you on 1000s of specialist parts, even if they are not listed on our website.
-
We can't find any results for ŌĆ£ŌĆØ.
Batch manufacturing in specialty chemicals lives at the intersection of complex chemistry, tight quality windows, and unforgiving economics. A single off-spec batch can mean wasted high-value raw materials, missed orders, and weeks of root-cause analysis. As a power system specialist who spends a lot of time in these plants, I see a common pattern: the technical conversation focuses on reactors, analytics, and automation, while the less glamorous foundations such as recipe management and power reliability quietly determine whether the fancy equipment actually delivers.
This article looks at batch control systems for specialty chemicals through the lens of recipe management and operational reliability. It brings together guidance from ISA-88, case studies from automation providers, process safety lessons from AIChE, optimization work from advanced control and AI firms, and KPI best practices from batch software vendors. The goal is to help you answer three very practical questions:
What does robust recipe management really look like in a specialty chemical plant? How can a batch control system shorten cycle time and improve yield without compromising safety? And how do you keep all of this resilient in the real world, where power disturbances, analyzer failures, and recipe changes are routine?
Throughout, I will also highlight where UPS systems, inverters, and power protection equipment make the difference between a controlled deviation and a ruined campaign.
Specialty chemical plants typically run smaller, higher-value batches with tight specifications and complex formulations. Factry notes that these batches are highly sensitive to variation in raw materials, operator actions, and timing, and that many plants still lack detailed, batch-level insight into what actually happened during each run. When you layer in energy-intensive heating, cooling, and mixing, the cost of poor control is not only scrap but also excessive utility consumption.
AIChEŌĆÖs Chemical Engineering Progress points out that in 2014 roughly $345.5 billion in specialty chemicals were produced using batch or semibatch processing, and a significant portion of that volume runs in tolling facilities. These tollers must constantly switch products, feedstocks, and processing conditions, which raises the risk of adverse reactions and equipment incompatibilities if process safety and recipe management are weak.
On the performance side, Burns & McDonnell emphasizes that automated batch processing improves efficiency, quality, waste, and safety by tightening recipe control and data logging. Imubit reports that modern advanced process control and AI can increase production in processing plants on the order of ten to fifteen percent by reducing cycle times and pushing operations closer to the true equipment limits while respecting safety margins.
Against that backdrop, a ŌĆ£recipeŌĆØ is no longer a Word document with steps like ŌĆ£heat to temperature and hold.ŌĆØ It is an executable specification tying together equipment phases, setpoints, tolerances, interlocks, data capture, and exception handling. When that specification is handled well, you get reproducible ŌĆ£golden batchesŌĆØ; when it is handled poorly, you get variability, frequent operator workarounds, and brittle automation that falls apart when the power flickers.
E Tech Group describes ISA-88 (often written S88) as the foundational framework for batch control in pharmaceuticals, and its concepts apply equally in specialty chemicals. S88 defines a modular way to describe equipment (units, equipment modules, control modules) and procedures (processes, unit procedures, operations, phases). The important point is that you build recipes out of reusable building blocks rather than one-off sequences for each product.
YokogawaŌĆÖs batch implementation paper goes further and warns that S88 provides the ŌĆ£goalŌĆØ but not the ŌĆ£map and compass.ŌĆØ The standard clarifies terminology such as unit supervision, recipe management, and production information management, but it does not tell you how to design and implement. From hard-earned project experience, the author stresses that a successful methodology always starts with thoroughly understanding the process, then designing, and only then implementing.
In practice, that means doing the unglamorous work before you write a single line of batch logic: studying P&IDs, mapping every transfer path, listing all roles of each vessel and valve, and talking to operators about the non-standard, exception-heavy batches. In many plants I visit, a single tank might be a raw material feed on Monday, a heel tank on Tuesday, and a cleaning solution mixer on Wednesday. If that reality is not reflected in your equipment and recipe models, your later automation will constantly fight the plant.
ISA-88 and modern batch systems let you transform free-form paper or spreadsheet recipes into structured, validated procedures. The Yokogawa methodology highlights several disciplines that consistently separate successful recipe management from painful projects.
First, everyone must agree on the recipe before you automate it. In many plants, quality, R&D, and operations all insist they ŌĆ£know how to make the product,ŌĆØ yet they disagree on details such as heat-up rates, addition order, or acceptable deviations. Automation forces these differences into the open. The vendor can advise based on S88 and control system capabilities, but the owner must own the final procedure and resolve internal conflicts.
Second, you need a written, peer-reviewed batch design that goes beyond the sequence of steps. The better projects document not only unit procedures and operations, but also preconditions to start each recipe, state transitions, alarm and exception handling, operator roles, batch reporting requirements, interfaces to MES and ERP, recovery after power loss, and constraints from environmental permits. Yokogawa describes formal ISO-style processes that separate functional design, detailed design, and implementation, with peer reviews and internal testing at each stage.
Third, involve operators early. The NovaTech D/3 and FlexBatch implementation at KratonŌĆÖs Savannah facility, as reported in ISA InTech, shows what this looks like when done well. The plant integrated ŌĆ£paperless proceduresŌĆØ so that manual and automated tasks live in the same batch programs, with SOP-like checklists on tablets and control room screens. Operators helped design displays and procedures, and the system records who did what and when. This did not replace operators; it removed ambiguity and timing variability, which had been driving out-of-spec batches even though the plant already had basic automation.
From a power reliability point of view, structured recipe management also makes it easier to design selective ride-through strategies. When you know precisely which units, phases, and data stores are critical to batch integrity, you can prioritize UPS-backed power to batch controllers, historian servers, analyzers, and key I/O rather than trying to put the entire plant on battery.
Consider a specialty resin that currently runs using a mix of paper instructions and operator experience. Today the steps might be loosely described as charging monomer and solvent, heating to a target temperature, gradually feeding initiator, holding for conversion, and then cooling and transferring.
An S88-based recipe would break this into standardized unit procedures such as ŌĆ£Charge,ŌĆØ ŌĆ£Heat and React,ŌĆØ ŌĆ£Feed Initiator,ŌĆØ ŌĆ£Hold and Monitor,ŌĆØ and ŌĆ£Cool and Transfer,ŌĆØ each composed of operations and phases tied to equipment modules. The ŌĆ£Heat and ReactŌĆØ phase would specify not only the target temperature but also allowable ramp rate, overshoot limits, interlocks to cooling water, and the required sample and lab analysis points. The ŌĆ£Feed InitiatorŌĆØ phase would codify timing and rate limits, agitation speed requirements, and what to do if a power dip interrupts the feed phase.
If every resin variant follows a similar structure, engineers can reuse and maintain code far more easily, operators quickly understand the state of a batch at a glance, and deviations become traceable in the batch record. KratonŌĆÖs experience with standardized FlexBatch recipes shows that this kind of structure improves repeatability and shortens cycle time because the system starts and stops heating, holds, and transfers consistently, rather than depending on who is on shift.
A simple way to think about maturity is shown below.
| Aspect | LowŌĆæmaturity behavior | HighŌĆæmaturity batch control behavior |
|---|---|---|
| Recipe format | FreeŌĆæform documents and operator habits | S88ŌĆæstructured control recipes with unit procedures, operations, and phases |
| Change control | Informal changes, tribal knowledge | Formal management of change with impact analysis and revalidation |
| Operator involvement | Automation handed over after coding | Operators coŌĆædesign displays and procedures, with training embedded in the tools |
| Power reliability design | Generic UPS sizing, based on rules of thumb | UPS, inverters, and backup supplies sized around critical recipe and data paths |
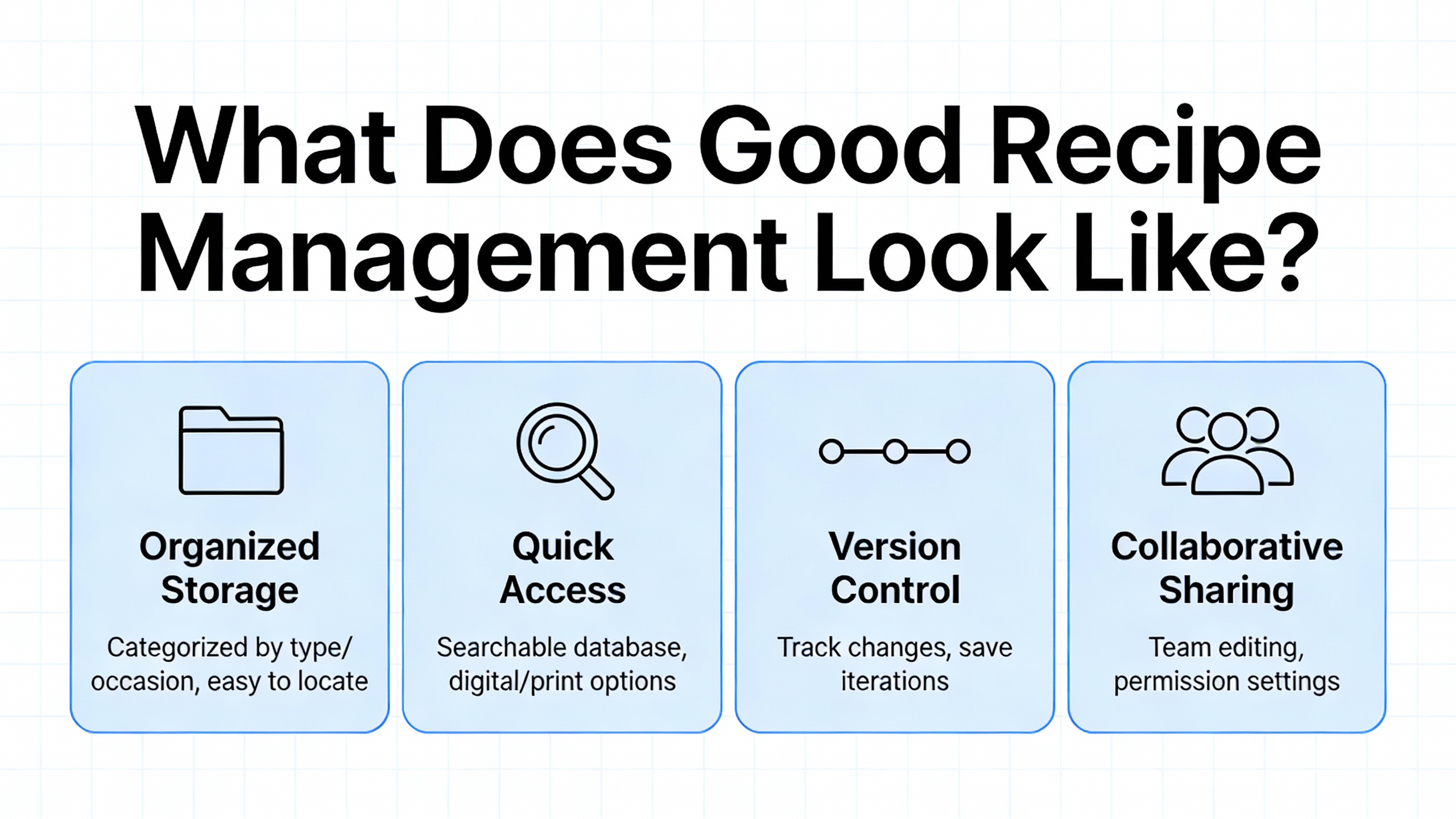
Once recipes are structured and repeatable, the next question is how to run them faster and more efficiently without compromising quality or safety.
A long-standing problem in batch plants is that end points and holds are often set conservatively. Fixed times and generous safety margins protect quality but waste capacity. An ISA blog on optimizing batch end points suggests using analyzers and profile slopes to make smarter, data-driven decisions.
The idea is straightforward. Instead of running a reactor for a fixed eight hours, you monitor a key concentration or conversion over time. Near the end of the batch, you look at the slope of that profile and infer how much more product you will make if you extend to the next analysis point. You also calculate the extra raw material and utility cost and compare that to the value of releasing the vessel sooner to start the next batch. If the incremental profit from extra product is lower than the value of regained capacity, you end the batch; if not, you extend.
The same source describes fed-batch reactors where the slope of concentration is used as a control variable, with operating conditions adjusted so that the current batch profile matches the plantŌĆÖs historical ŌĆ£bestŌĆØ batch. In bioreactors, online analyzers for cell concentration and substrate enable control of nutrient ratios and cell growth rate, rather than only controlling temperature and pH.
These strategies require reliable analyzers or inferential measurements and robust exception handling when data are missing or noisy. They also assume your batch controller and data acquisition remain stable through power dips. In several plants I have worked with, operators have been reluctant to trust slope-based optimization because every time the analyzer or historian PC rebooted after a voltage sag, the batch optimization screen would freeze. After those outages, they reverted to fixed-time recipes. UPS-backed power for analyzers, control servers, and switches is what turns slope-based control from a laboratory idea into a dependable production tool.
Imubit and other AI APC providers frame batch optimization as a multivariable control problem. Traditional PID loops are tuned for single variables and often use conservative setpoints because gains change drastically across batch phases, and operators do not have a predictive view of how aggressive they can be without crossing constraints.
According to Imubit, AI-enabled control treats the batch as an integrated system, jointly optimizing temperature, pressure, flows, and quality indicators. Machine-learning models predict batch end quality in real time, which allows termination as soon as specifications are achieved rather than at a fixed time. Reinforcement learning controllers can gradually learn better operating policies batch by batch, respecting safety constraints.
Reported outcomes in chemical and polymer manufacturing include cycle time reductions in the tens of percent, enough in some cases to move from three batches per day to four while staying within the same asset base. That is a throughput increase on the order of one third without adding reactors or utilities. McKinseyŌĆÖs work cited by Imubit suggests that AI-enabled advanced control can raise production roughly ten to fifteen percent in process industries by closing the gap between current operating practice and the equipmentŌĆÖs true capability.
Chemcopilot describes complementary use of digital twins and AI models to simulate and optimize processes, running thousands of ŌĆ£virtual experimentsŌĆØ in seconds. These tools extend traditional statistical process control and chemometrics by tying together lab data, online spectroscopy, and plant sensors in a closed feedback loop between R&D and manufacturing.
All of this is only valuable if the underlying recipe and batch structures are sound. AI is powerful at exploring operating space but does not replace the need for S88-based recipe modularity, well-defined exception logic, and process safety safeguards. It is also power-sensitive: controllers, GPU servers, and storage need clean, continuous power. I have seen high-performance AI servers fail in the field because they were added to existing control rooms without revisiting UPS capacity, branch circuit loading, or grounding; a brief voltage sag would trip the AI box while the legacy control system continued riding through on its own UPS. If you intend to lean on AI for end-point decisions, design the power system so that it is at least as resilient as the basic controllers.
Advanced reactor hardware strongly influences what batch control and recipes can achieve. A specialty chemical case study from Stalwart International reports that implementing a modern reactor system with automated temperature control, high-performance mixing, and real-time monitoring shortened batch time by about thirty percent, improved product homogeneity by twenty percent, and cut downtime by forty percent. These gains came from better heat transfer, more uniform mixing, and fewer manual interventions.
Stalwart also highlights the role of automation, predictive maintenance, and digital twins for reactors. Digital models that mirror real equipment let engineers test new recipes, conditions, and cleaning cycles virtually before touching the plant. This aligns with the digital twin use described by Chemcopilot, where virtual reactors are continuously synchronized with sensor data to catch fouling, feed changes, or impending thermal runaway early.
The ŌĆ£golden batchŌĆØ concept, frequently used in AI and APC discussions, comes down to learning from your best historical runs and teaching the control system to replicate those trajectories. S88-based batch records, with detailed time-stamped data, are the raw material for that learning. Stable power to sensors and recorders is again a prerequisite: if your data streams are full of gaps from power blips, neither traditional SPC nor advanced AI models will trust the dataset enough to guide optimization.
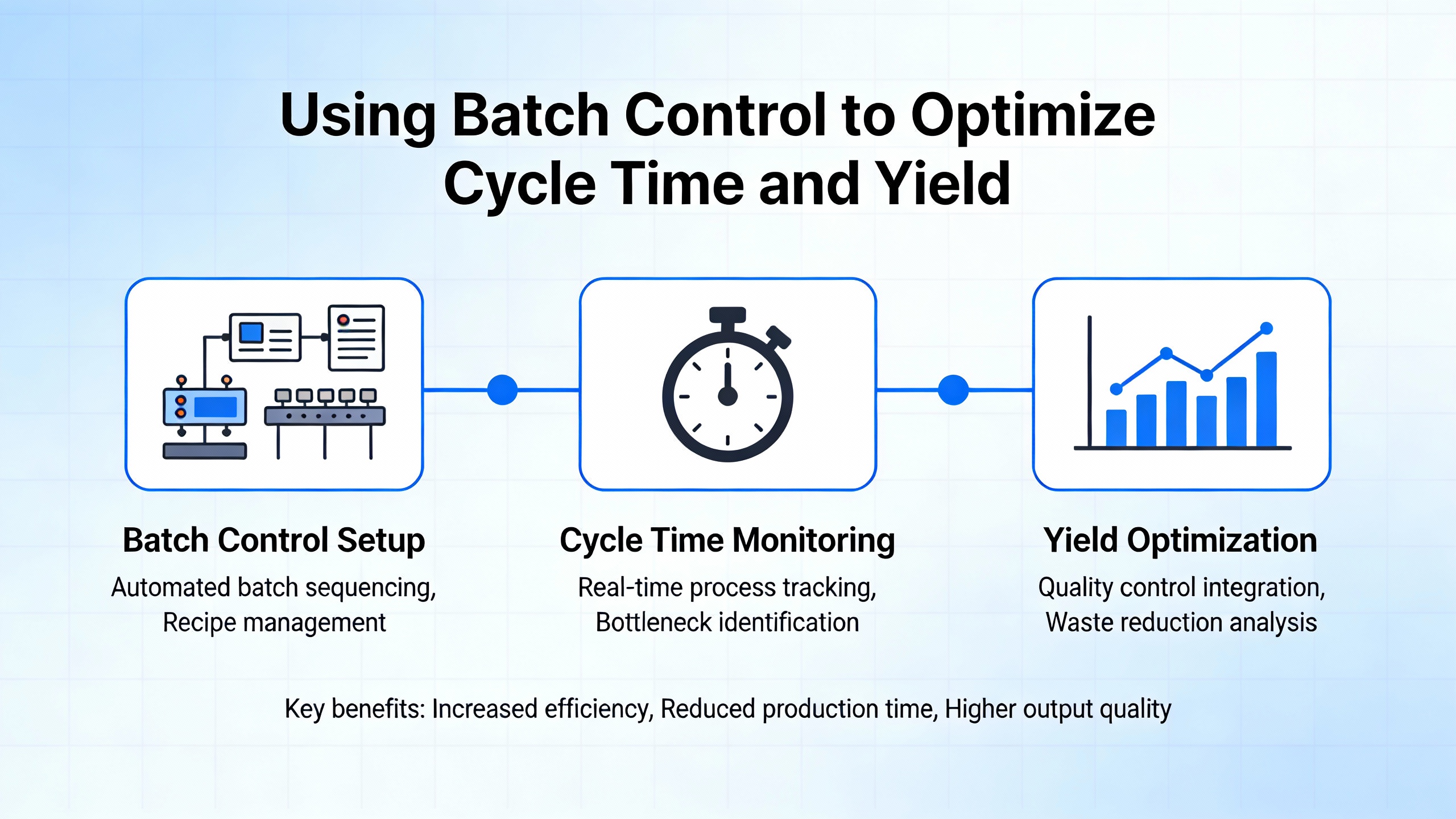
AIChEŌĆÖs Chemical Engineering Progress article on batch tolling underscores how dynamic and risk-intensive tolling operations can be. Toll manufacturers may run more than one hundred new product trials or processes per year, each with different raw materials, hazards, and equipment configurations. OSHAŌĆÖs Process Safety Management regulation is performance-based, meaning facilities must meet its fourteen elements but can choose how to do so. For batch tollers, the real challenge is managing process safety information and hazard analysis across many changing recipes.
The article recommends a structured screening process for every new or modified product, starting with collecting detailed reaction chemistry, safety data sheets, combustible dust information, reaction kinetics, and analytical methods from the customer. A first-level evaluation checks whether the product can be safely tested in the lab. If that passes, a lab study examines the reactions and economics and feeds into a more comprehensive plant-level assessment.
At the plant level, safety and engineering personnel assess whether existing equipment, relief devices, fire protection, foam systems, and emergency response procedures can handle the new chemistry. Several examples show the value of this discipline. One toller refused a product when the main raw material was incompatible with Hastelloy patches in a glass-lined reactor. Another declined to handle a pyrophoric material because existing equipment could not safely manage it. In a third case, the team discovered that making a customerŌĆÖs catalyst in-house would generate hydrogen gas that the facility was not equipped to handle, so they declined the job.
Once a product is deemed feasible, it should pass through formal management of change, including a process hazard analysis. For batch vessels, AIChE suggests using ŌĆ£baselineŌĆØ PHAs for equipment and then reviewing new processes against them, rather than doing full PHAs from scratch for every new campaign. This is especially helpful in plants that run many different recipes in the same vessels.
All of these actions depend on accurate, accessible documentation and on reliable batch records. If the plant experiences frequent power disruptions that reset controllers, leave batches incomplete in the historian, or corrupt trend data, it becomes much harder to demonstrate compliance or revisit incident histories. From a power system perspective, highly automated tollers should treat PSM-critical control and data infrastructure as life-safety-grade loads, backed by appropriately sized UPS, clean grounding, and selective coordination with upstream protective devices.
YokogawaŌĆÖs real-world lessons emphasize that exception handling is ŌĆ£the hard partŌĆØ of batch and must not be an afterthought. It covers what happens when a valve fails to move, an analyzer sample is rejected, a temperature limit is exceeded, or the operator cannot respond to a prompt. Poorly defined exception logic leads to unplanned batch aborts, unsafe conditions, or improvised operator overrides.
Batch reporting is similarly not an add-on. E Tech Group notes that S88 compliance supports regulatory requirements such as FDA and cGMP by generating structured batch records suitable for audits and investigations. Mar-KovŌĆÖs KPI guidance adds that metrics such as first-pass yield, scrap and rework rates, out-of-spec batches, and deviation closure time are essential for continuous improvement.
To maintain data integrity, many plants move toward electronic batch records with time-stamped procedural steps, automated data capture, and built-in approvals. KratonŌĆÖs use of paperless procedures, as described by ISA InTech, is a concrete example. Operators execute both manual and automated tasks within the batch system, which automatically records who did what and when, improving traceability and enabling faster root-cause analysis.
Once again, the weakest link can be power. If a ten-second voltage sag resets the batch reporting server or corrupts an in-flight batch record, the plant may need to reconstruct data from logs and memory, which regulators justifiably view with skepticism. In my reliability assessments, I routinely find that plants protect the DCS controllers but neglect the historian, report servers, or network switches, assuming these are ŌĆ£ITŌĆØ loads. For a batch environment, they are part of the recipe execution and must be on conditioned, backed-up power.
From the perspective of batch reliability, power systems have two critical jobs: maintaining control continuity through short disturbances and ensuring orderly shutdown and recovery when outages last longer than backup autonomy.
Short sags and momentary interruptions can cause PLCs, DCS controllers, analyzer PLCs, and industrial PCs to reboot. If that happens mid-batch, the plant may lose the exact step, phase, or timing context, even if power returns in a second. For high-value specialty batches, it is rarely acceptable to ŌĆ£guess and continue.ŌĆØ UPS systems on these loads provide ride-through, keeping controllers and key network gear powered while upstream feeders clear faults or generators start. Many specialty plants can tolerate a brief process upset, such as a few seconds of lost agitation, but cannot tolerate a reset of the batch recipe engine.
Longer outages raise a different question: how much of the plant do you keep alive, and for how long? Full facility backup with diesel generators and large central UPS systems is one strategy, but many specialty chemical sites instead deploy selective protection. Critical batch control, safety instrumented systems, analyzers, and batch data infrastructure get longer-duration UPS and possibly local generator backup. Less critical loads, such as non-essential HVAC or general lighting, may drop earlier.
From a recipe management standpoint, what matters is that the system knows when it is safe to resume, when to abandon, and how to record what happened. This requires coordination between power engineers and control engineers. For example, you may design your UPS runtime so that controllers and historians can survive long enough to bring the batch to a defined safe state, commit all data, and then shut down gracefully if the generator fails to start.
Well-designed inverters and static transfer switches can also protect sensitive control loads from harmonics, switching transients, and motor starts elsewhere on the plant bus. These disturbances rarely show up in generic uptime statistics but can be the root cause behind random controller resets and unexplained communication errors. In that sense, a clean, reliable power supply is as much a part of recipe management as your S88 implementation; it is the difference between a recipe you can trust and one that behaves differently on stormy days.
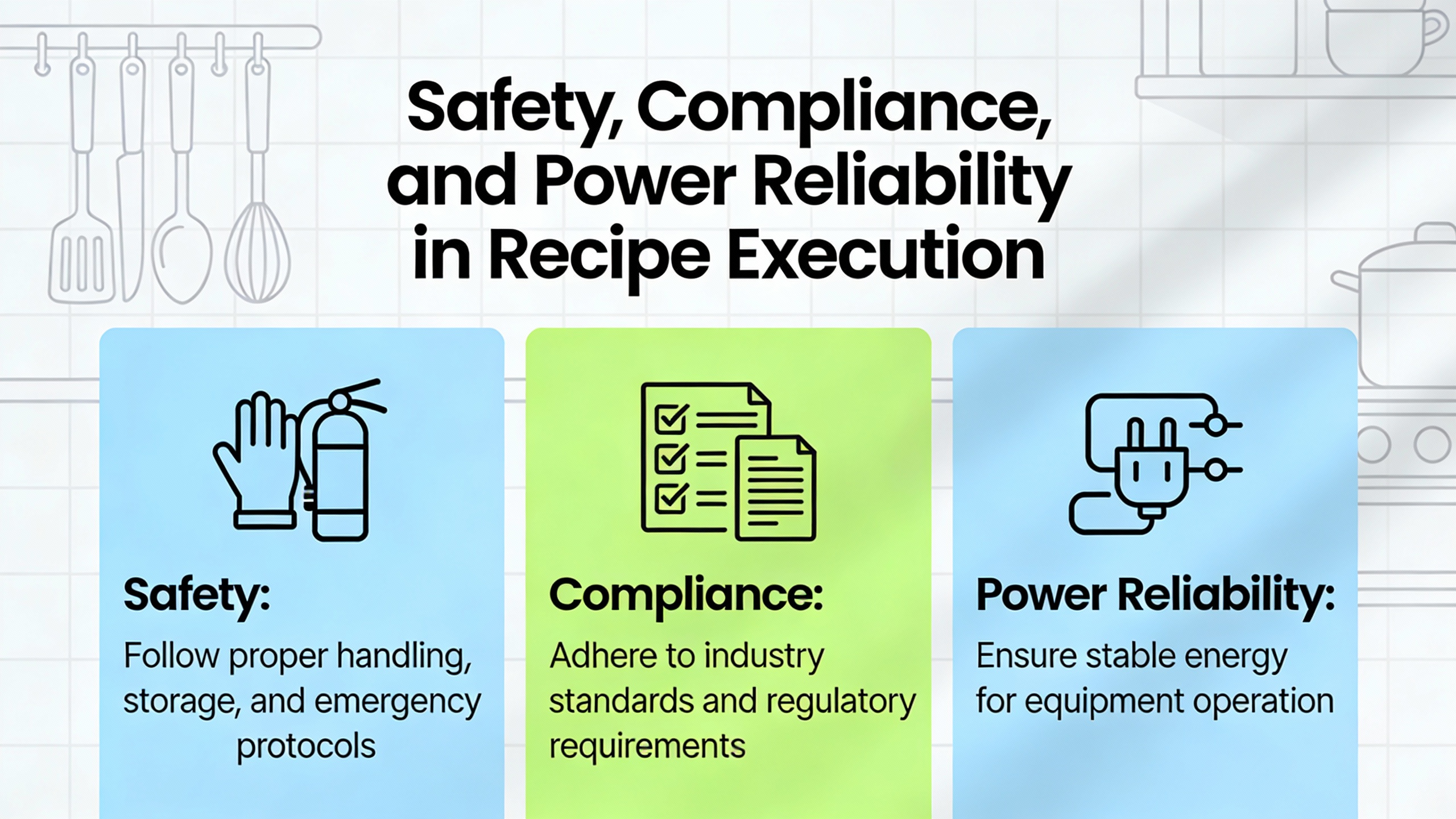
Most specialty chemical plants cannot afford a ŌĆ£big bangŌĆØ replacement of their batch systems. Burns & McDonnell advocates a structured, phased automation roadmap: assess current operations for high-impact opportunities, verify feasibility within budget, align stakeholders early, and then scale automation without disrupting ongoing production.
PharmaSalmanacŌĆÖs review of trends in chemical process development complements this with several relevant directions. Continuous processing and modular plant systems are gaining ground, especially where they can reduce cycle times, shrink equipment footprints, and simplify quality control. However, for many high-value, lower-volume specialties, batch remains the practical mode, and modular design principles can still help. Building lab equipment and production units around standardized modules makes it easier to scale recipes and reuse control strategies.
Quality by Design approaches, also highlighted in that article, align naturally with S88 batch control and recipe management. Defining critical quality attributes, understanding process variability, and controlling parameters within proven design spaces are all easier when you have well-structured control recipes and detailed batch records. Digitization and automation then form a physicalŌĆōdigitalŌĆōphysical loop: sensors collect data, analytics and AI propose adjustments, and batch equipment executes them under defined rules.
Mar-Kov recommends using KPIs such as overall equipment effectiveness, batch cycle time, changeover time, first-pass yield, and deviation metrics to drive continuous improvement. These indicators feed the ŌĆ£CheckŌĆØ stage of the PlanŌĆōDoŌĆōCheckŌĆōAct cycle and should rely on automated data capture from MES, SCADA, ERP, and LIMS systems.
In practice, a pragmatic roadmap for recipe-focused batch modernization in specialty chemicals often looks like this. You stabilize the power environment for control and data systems so that future automation has a solid foundation. You adopt S88-based recipe structures, starting with a pilot line or high-impact product family. You formalize process safety screening and PHAs around that equipment. You then layer in paperless procedures, batch reporting, and basic KPI dashboards. Once that foundation is delivering consistent golden batches, you selectively deploy advanced control, end-point optimization, or AI where the economics justify it.
Throughout, power system decisions run in parallel. Every time you add new critical batch or data functionality, you revisit UPS sizing, inverter loading, grounding, and generator coordination. The goal is not just ŌĆ£more automation,ŌĆØ but automation that stays online, captures complete records, and recovers gracefully when the grid misbehaves.
Work summarized by The Chemical Engineer and PharmaSalmanac shows that continuous processing can roughly halve operating costs and significantly improve yield and waste in some cases, especially for high-volume products and suitable chemistries. However, the same sources note that most complex, lower-volume specialty and pharmaceutical processes remain batch because of slower reactions, flexible product portfolios, and existing infrastructure. A practical approach is to strengthen batch recipe management and control now, while evaluating specific unit operations for potential continuous or intensified alternatives where the chemistry and volumes justify it.
Advanced process control and AI, as described by Imubit and Chemcopilot, sit above your core S88 batch control. They optimize setpoints, trajectories, and end points within the bounds set by recipes, safety constraints, and regulatory requirements. If your recipes are inconsistent, your data is incomplete, or your power system lets controllers and historians reset mid-batch, AI will mostly learn noise. Conversely, if you have clean, consistent batch records and reliable power, AI can help you move more of your operation toward golden-batch performance and shorten cycle times without sacrificing quality.
Robust batch control and recipe management are not just software projects; they are engineering disciplines that connect chemistry, safety, operations, and power reliability. When you align those disciplines, your plant stops fighting its automation and starts using it as a competitive advantage.





Leave Your Comment